Robert J. Carpenter, MD, Michael F. Angel. MD, and Raymond F. Morgan, MD
Rochester, New York
There is ample evidence of the involvement of free radicals in mediating skin flap necrosis. Dimethyl sulfoxide (DMSO) is a well-tolerated, safe drug that is a powerful scavenger of the hydroxyl free radical. The current study investigated the effect of DMSO on the survival of 9 x 4 cm skin flaps based on the epigastric vessels subjected to primary venous occlusion. Forty-seven skin flaps were elevated and the epigastric vein was occluded by a microvascular clamp for 8 hours. Group 1 received DMSO (1.5nbsp;gm/kg) intraperitoneally at reperfusion. Group 2 received saline solution, group 3 received DMSO at reperfusion and every day for 5 days, group 4 received DMSO preoperatively and then as in group 3, and group 5 was the saline solution control for groups 3 and 4. DMSO did not increase percent flap survival when given as a single dose at reperfusion (40.6% ± 42.7%) compared with saline solution (33.7% ± 41.2%). When DMSO was continued in the postoperative period, group 3 (86.2% ±nbsp;25.8%) and group 4 (78.0% ± 32.5%) had significantly better survival than the saline solution control group (32.6% ± 39.8%) (p < 0.01 and p < 0.03, respectively). There was no significant difference between groups 3 and 4. DMSO administered at reperfusion and postoperatively for 5 days significantly increased flap survival. It is hypothesized that this occurs through scavenging deleterious free radical species. This effect may have clinical significance. (Otolaryngol Head Neck Surg 1994:110: 228-31.)
Increasing interest in the mechanisms of ischemia/reperfusion injury has been sparked by technical advances in plastic surgery such as free tissue transfer, limb replantation, and the use of long vascular pedicles. The production of oxygen-derived free radicles (OFRs) is widely recognized as a major cause of reperfusion injury in many organ systems. 1, 2 Importantly, survival of both random and axial skin flaps has been reported to be heavily influenced by OFR production. 3-6 Agents that block the formation of OFRs (allopurinol,7, 8 soybean trypsin inhibitor9) as well as OFR scavengers (superoxide dismutase,3 mannitol,8, 9 DMSO,9 desferoxamine,5 and N-2-mercaptopropioniglycine9) have all been shown to increase survival of skin flaps.
Dimethyl sulfoxide (DMSO) was first synthesized in 1866. It has a pyramidal structure, with sulfur at the center and two methyl groups and an oxygen atom (with a nonbonding electron pair) at the apices. Because of this structure, DMSO has exceptional solvent properties. The free electron pair allows it to participate in the transfer of electrons and makes DMSO a free radical scavenger with a high degree of specificity for the damaging hydroxyl radical. Investigators have found that DMSO lessens reperfusion injury in a variety of tissues, including intestine,10, 11 kidneys,12 muscle,13 and skin. 8
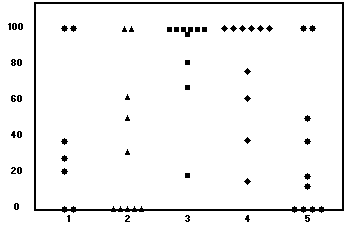
![]()
Fig. 1. DMSO study: Percent flap survival. Group 1,
reperfusion; group 2, control; group 3, reperfusion + 5
days; group 4, pretreatment + 5 days; and group 5,
control.
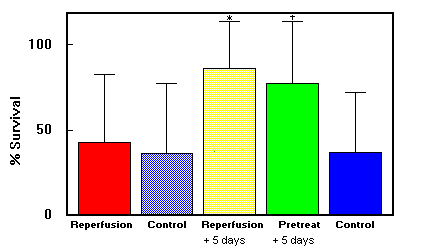
Fig. 2. DMSO study: Mean (±SD) survival in skin flap groups.
*Group 3 had better survival rate than group 1 (p <
0.01>. tGroup 4 had a better survival rate than group 5 (p < 0.03).
Early studies with DMSO demonstrated enhanced survival in a dorsal flap model,14 which was an an axlal flap with a random extention. Others have confirmed this finding. 15 Narayanan et al. 9 found that DMSO enhanced flap survival in a different model. Epigastric island flaps were subjected to 15 hours of total primary ischemia. Skin flap survival increased from 13% to 63% with the administration of DMSO.
The current study evaluated the effectiveness as well as optimal timing of administration of DMSO in a model of primary venous occlusion.
Female Sprague/Dawley rats (n = 47), weighing 230 to 249 gm each, were used. They were housed individuaIly and given water and rat chow ad Iibitum. Animals were humanely cared for in compliance with The Principles of Laboratory Animal Care, formulated by the National Society for Medical Research, and the Guide for the Care and Use of Laboratory Animals, prepared by the National Academy of Sciences and published by the National Institutes of Health. With the animals under pentobarbital anesthesia (35 mg/kg intraperitoneally), a 9 x 4 cm abdominal flap, based on the left epigastric neurovascular pedicle, was elevated as previously described by others16 and resutured to its bed. The medial border of the flap formed a line runing from xiphoid to pubis. The vein of the flap was then occluded with a microvascular clamp for 8 hours. After removal of the clamp, the vein was observed to assure the return of venous blood flow. DMSO (Sigma Chemical, St. Louis, Mo.) was given intraperitoneally (1.5 mg/kg) according to the following protocol:
Group 1 (reperfusion only): DMSO was given at reanesthesia for removal of the microvascular clamp on the vein (n = 7)
Group 2 (controls for group 1): received 0.2 ml normal saline solution intraperitoneally as in group 1 (n = 10)
Group 3 (reperfusion + 5 days): DMSO was given as in group 1 and then continued daily for 5 days (n = 10)
Group 4 (pretreatment + reperfusion + 5 days): DMSO was given before initial flap elevation, then as in group 3 (n = 10)
Group 5 (controls for groups 3 and 4): 0.2 ml normal saline solution was given on the same schedule as in group 4
Flap viability was assessed on postoperative day 5. The area of survival was calculated using a sonic digitizer and expressed as a percent of the original total flap area as previously described. 17 The Wilcoxon-Mann-Whitney two-sample test and the Kruskal-Wallis K-sample test were used to determine significance.
Data are summarized in Figs. 1 and 2 and Table 1. DMSO showed no increase in flap survival when given as a single intraperitoneal dose at reperfusion (40.6% ± 42.7%) when compared with saline solution (33.7% ± 41.2%). Continuing DMSO administration postoperatively increased the percentage of flap survival significantly (86.3% ± 25.8%) compared with control (32.6% ± 39.8%; p < 0.01), Pretreatment with DMSO before the onset of ischemia (78.0% ± 32.5%) did not confer survival benefit compared with beginning treatment at reperfusion.
| Group no. | Average percent flap survival | P value |
| 1 | 40.6 ± 42.7 | > 0.05 |
| 2 | 33.7 ± 41.2 | NS |
| 3 | 86.2 ± 25.8 | 0.011 |
| 4 | 78.0 ± 32.5 | 0.026 |
| 5 | 32.6 ± 39.8 | NS |
DMSO has been extensively studied by both chemists and physicians. It is a small (78 molecular weight) molecule that readily crosses the membranes of cells and organelles as well as intact skin. DMSO scavenges the hydroxyl radical (OH), one of the most toxic by-products of superoxide radical formation. Schlafer18 noted DMSO to be an ideal OH scavenger because it (1) is specific for OH, (2) reaches the sites of OH generation (down to the mitochondrial level), and (3) lacks cellular toxicity.
DMSO also causes vasodilation in small and intermediate vessels, which can be blocked by the administration of antihistamines. 14, 15
DMSO has shown mixed results in increasing skin flap viability when used topically. 14 However, when administered either intraperitoneally or intravenously, DMSO increased survival in both random and island skin flap models. 9, 20, 21
The current model is an axial flap with a random extension. In addition, it received a primary ischemic insult by venous obstruction as first described by Harashina et al. 16 In this model, therefore, several processes are occuring. The distal part of the flap is in a partially ischemic state, which is a source of free radical production. 22 Once venous obstruction occurs, further stresses are placed on the entire flap: hydrostatic forces increase, and ischemia occurs with production of free radicals and other mediators of tissue destruction. 23, 24
The current study failed to show a benefit when DMSO was given as a single dose at reperfusion. This is in contrast to the experience of Narayanan et al.,9 who found significant survival benefit from a single dose of DMSO. This discrepancy between studies is not unexpected. The dose of DMSO used in Narayanan et al.'s work (5 gm/kg) was more than threefold our dose (1.5 gm/kg). In addition, the experimental models are different. The former study uses an axial flap that underwent 15 hours of primary arterial ishemia. Production of OFRs would be expected to be maximal during the period of reperfusion. In our study, by contrast, flaps suffered OFR production throughout the venous obstruction, reperfusion, and postreperfusion periods. From this scenario one would expect DMSO to be beneficial when given at these times of OFR production. Therefore, group 4, which received DMSO before the reperfusion and during the postreperfusion period, had significantly better survival than control animals. In this study, however, administration of DMSO before venous obstruction offered no additional benefit. This may be explained by hypothesizing that although OFRs are produced during primary venous obstruction, there are other, more important damaging processes (i.e., hydrostatic forces) that are unaffected by DMSO.
DMSO is a safe, well-tolerated drug. 6, 25 It has demonstrated beneficial effects in a number of models and organ systems. It significantly improves flap survival in this study and may have clinical significance.
- Bulkley GB. Pathophysiology of free radical-mediated reperfusion injury. J Vasc Surg 1987; 5:512.
- Angel MF. Ramasastry SS, Swartz WM, Basford RE, Futrell J. Free radicals: basic concepts concerning the chemistry, pathophysiology, and relevance to plastic surgery. Plast Reconstr Surg 1987; 79:990.
- Manson PN, Anthenelli RM, lm MJ, Bulkley GB, Hoopes JE. The role of oxygen-free radicals in ischemic tissue injury in island skin flaps. Ann Surg 1983; 1983:87.
- Huang L. Privalle CT, Serafin D, Klitzman B. Increased survival of skin flaps by scavengers of superoxide radical. FASEB J 1987; 69:469.
- Angel MF, Narayanan K, Swartz WM, et al. Deferoxamine increases skin flap survival: additional evidence of free radical involvement in ischemic flap surgery. Br J Plast Surg 1986; 69.469
- Kim YS, lm MJ, Hoopes J. The effect of a free radical scavenger, N-2-Mercaptopropionyiglycine, on the survival of skin flaps. Ann Plast Surg 1990; 25:15.
- IM MJ, Shen W, Pak CJ, Manson PN, Bulkley GB, Hoopes J. Effect of allolpurinol on the survival of hyperemic isIand skin flaps. Plast Reconstr Surg 1984; 73:276.
- Angel MF, Narayanan K, Swartz WM, Ramasastry SS, Basford RE, Futrell J. Augmentation of skin flap survival with allopurinol. Ann Plast Surg 1987; 18:494
- Narayanan K, lm MJ, Manson PN, Bulkley GB, Hoopes J. Mechanism and prevention of ischemia/reperfusion injury in isIand skin flaps. Surg Forum 1985; 36:593.
- Parks DA, Shah AK, Granger D. Oxygen radicas: effects on intestinal vascular permeability. Am J Physiol 1984; 247:167.
- Ravid M, Van-Dyk D, Berneim J. The protective effect of dimethyl sulfoxide in experimental ischemia of the intestine. Ann NY Acad Sci 1983; 411:131.
- Kedar I, Jacob ET, Bar-Natan N, Ravid M. DimethyI sulfoxide in acute ischemia of the kidney. Ann NY Acad Sci 1983; 411:131.
- Feller AM, Roth AC, Russell R. Experimental evaluation of oxygen free radical scavengers in prevention of reperfusion injury in muscle. Ann Plast Surg 1989; 22:131.
- Adamson JE, Hotton CE, Crawford HH, Ayers W. Studies on the action of dimethyl sulfoxide on the experimental pedicle flap. Plast Reconstr Surg 1989; 22:321.
- Haller J, Trachy, Cummings R. Effect of dimethyl sulfoxide on island flap perfusion and survival in rats. Arch Otolaryngol Head Neck Surg 1987: 113:859.
- Harashina T, Sawada Y, Watanabe S. The relationship between venous occlusion time in island skin flaps and flap survival. Plast Reconstr Surg 1977; 60:92.
- Nichter LS, Sobieski MW, Morgan RF, Rodeheaver G, Edlich R. Quantitation of skin flap survival: a computer based method. Plast Reconstr Surg 1984; 73:684.
- Schlafer M. Cardiac pharmacology of dimethyl sulfoxide and its postulated relevance to organ preservation in ischemic or hypoxic states. Ann NY Acad Sci 1983; 411:170.
- Koehnlein HE, Lemperle G. Experimental studies on the effect of dimethyl sulfoxide on pedicle flaps. Surgery 1970; 67:672.
- Arturson G, Khanna N. The effects of hyperbaric oxygen, dimethyl sulfoxide, and Complamin on the survival of experimental skin flaps. Scand J Plast Reconstr Surg 1970: 4.8.
- Grossman JAI, McGonagle BA, Dowden RV,Dinner M. The effect of hyaluronidase and dimethyI sulfoxide (DMSO) on experimental skin flap survival. Ann Plast Surg 1983: 11:223.
- Angel MF, Ramasastry SS, Swartz WM, Narayanan K, Kuhns DB, Futrell J. The critical relationship between free radicals and degrees of ischemia: evidence for tissue intolerance of marginal perfusion. Plast Reconstr Surg 1988; 81:233.
- Angel MF, Mellow CJ, Knight KR, Morgan RF, O'Brien McC. Biochemical analysis of acutely ischemic skin flaps. Curr Surg 1990: 47:272.
- Im MJ, Manson PN, Bulkley GB, Hoopes J. Effects of superoxide dismatase and allopurinol on the survival of acute island skin flaps. Ann Surg 1985; 201:357.
- Trice JM, Pinals RS. Dimethyl sulfoxide: a review of its use in the rheumatic disorders. Semin Arthritis Rheum 1985: 15:45.
From the Department of Surgery (Dr. Carpenter). University of Rochester School of Medicine; the Division of Plastic Surgery (Dr. Angel), The Johns Hopkins School of Medicine, and the Department of Plastic Surgery. (Dr. Morgan), University ofVirginia Health Sciences Center.
Received for publication March 12, 1993; revision received June 9, 1993; accepted June 17, 1993.
Reprint requests: Robert J. Carpenter, MD. Department of Plastic Surgery, The Johns Hopkins Hospital, 601 North Caroline St./McElderry 8152C, Baltimore, MD 21287.
Copyright © by the American Academy of Otoloryngology-Head and Neck Surgery Foundation, Inc.
0194-5998/94/$3.00 + 0 23/10/49496
DMSO wishes to thank to thank the publishers for their kind permission to put this document on our web site. Copyright for this article is retained by the publisher. Permission to copy any portion of this document must be obtained from the publisher.
© 2001-2022 All rights reserved