L Hideto Yoshikawa, MD
Division of Inherited Metabolic
Disorders, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Kodaira
Tokyo, Japan
Accepted for publication: March 16, 1991
AY-9944 and other cationic amphiphilic drugs (CADs) have been reported to cause a reduction of acid sphingomyelinase activity in fibroblasts and various tissues in the rat, and DMSO has been known to correct a partial deficiency of acid sphingomyelinase activity in Niemann-Pick type C (NPC) flbroblasts. Furthermore, in the present study which demonstrated that A Y-9944 and other CADs caused a marked reduction of cholesterol esterification in control fibroblasts and an excessive intracellular accumulation of unesterified cholesterol as in NPC cells, and that this reduction could partially be ,corrected by the addition of 2% DMSO to the medium. These characteristics of the drug-treated cells mimic the phenomena seen in NPC fibroblasts. Therefore, fibroblasts treated with CADs may be used as a model of drug-induced lipidosis of NPC. The effect of DMSO suggests the possibility of its usefulness in the treatment of NPC patients.
Niemann-Pick disease type C (NPC) is an inherited neuro-visceral lipid storage disorder, and the primary lesion of NPC is still elusive. 1 Biochemically, in NPC the acid sphingomyelinase activity is normal or only slightly decreased. Recently, it was reported that an excessive unesterified cholesterol is accumulated because the esterification of exogenously derived cholesterol is defective. 2, 3 A partial deficiency of sphingomyelinase is not considered to reflect the primary lesion of NPC. Therefore, NPC is now appropriately defined as unesterified cholesterol lipidosis rather than as sphingomyelin lipidosis. 1
On the other hand, AY-9944 (trans-1, 4-bis(2-chlorobenzylaminoethyl) cyclohexane dihydrochloride),4 a cationic amphiphilic drug (CAD), is a hypocholesterolemic drug which acts through the inhibition of 7-dehydrocholesterol reductase. It was reported that AY-9944 and other CADs caused a rapid and dose dependent reduction of acid sphingomyelinase activity in normal human fibroblasts without changing the activities of other lysosomal hydrolases. 5 Also, AY-9944 produced the condition in the animals which exactly mimicked the situation in patients with Niemann-Pick disease. 6 On the contrary, treatment with 2% dimethyl sulfoxide (DMSO) caused a marked increase of sphingomyelinase activity in normal and NPC fibroblasts. 7 In light of these observations, we investigated the effects of the drugs, especially of the CADs and of DMSO and its interactions, on cholesterol esterification and discussed the possibility of a drug-induced NPC model and the drug treatment of NPC by DMSO.
Materials
[1,2-3H] Cholesterol (53.0 Ci/mmol) and [9,10-3 H] oleic acid (5.3 Ci/mmol) were purchased from New England Nuclear (Boston, MA, USA). Filipin and human low-density-lipoprotein (LDL) were obtained from Sigma (St Louis, MO, USA). Lipoprotein-deficient bovine serum (LPDS) was obtained from Sankyo Co. (Tokyo, Japan). Fetal bovine serum and penicillin/streptomycin solution were purchased from GIBCO (Grand Island, NY, USA) and Dulbecco's modified Eagle medium (DME medium) from Nissui Pharmaceutical (Tokyo, Japan). AY-9944 was kindly supplied by Dr. Dvornik (Ayerst Laboratories, Montreal, Canada). Chloroquine diphosphate and dibucaine hydrochloride were obtained from Sigma. Imipramine hydrochloride, desipramine hydrochloride, and clomipramine hydrochloride were gifts from CIBA Geigy Japan Co. (Takarazuka, Japan). Mianserin hydrochloride was a gift from Sankyo Co. (Tokyo, Japan). Lofeptamine hydrochloride and trimipramine maleate were provided by Daiichi Pharmaceutical Co. (Tokyo, Japan) and Shionogi Pharmaceutical Co. (Osaka, Japan), respectively. Chlorpromazine hydrochloride was purchased from Wako Pure Chemical Co. (Osaka, Japan). W-7(N-(6-aminohexyl)5-chloro-1-naphthalene sulfonamide) and W-5(N-(6-aminohexyl)-1-naphthalene sulfonamide) were from Seikagaku Kogyo Co. (Tokyo, Japan).
Cell Culture
Control
and NPC fibroblast cultures were derived from superficial
skin biopsies of volunteers and diagnostically confirmed
patients. Cells were seeded in single-chamber Labtek
(Miles) slide chambers (50,000 cells/chamber) for cytochemical
studies, and in 60 mm diameter culture dishes for
other experiments. Cells were grown in DME medium supplemented
with 10% (v/v) fetal bovine serum, penicillin (100 units/ml)
and streptomycin (100 ![]() g/ml)
in humidified C02 at 37°C. The details
of each experiment are described in the Figures
and Tables.
g/ml)
in humidified C02 at 37°C. The details
of each experiment are described in the Figures
and Tables.
Lipid analysis
The
cells were harvested by trypsinization and further washed
twice with phosphate-buffered saline (PBS). The final
pellet was homogenized in distilled water (0.6 ml)
by sonication. Protein was measured on a suitable aliquot
(usually 10 ![]() l)
by the method of Lowry et al. 8
Lipids were studied on a 0.5 ml portion of the
total cell homogenate. Lipid extraction was performed
with the method of Folch et al. 9
The lipid extract was analyzed by thin-layer chromatography
on silica gel plates (Wako Pure Chemical Co.) using
hexan-ethylether-glacial acetic acid 90:10:1 (by vol.)
as solvent system. After visualization with iodine vapor,
the relevant lipids were scraped from the plates and
their radio activity was measured by liquid scintillation
spectrometry in 5 ml of Aquasol.
l)
by the method of Lowry et al. 8
Lipids were studied on a 0.5 ml portion of the
total cell homogenate. Lipid extraction was performed
with the method of Folch et al. 9
The lipid extract was analyzed by thin-layer chromatography
on silica gel plates (Wako Pure Chemical Co.) using
hexan-ethylether-glacial acetic acid 90:10:1 (by vol.)
as solvent system. After visualization with iodine vapor,
the relevant lipids were scraped from the plates and
their radio activity was measured by liquid scintillation
spectrometry in 5 ml of Aquasol.
Cytochemical staining of unesterified cholesterol
Cells
were plated in a single glass slide chamber (50,000
cells per chamber) and grown for 3 days in DME medium
supplemented with 10% FCS. Fresh FCS-supplemented medium
with the presence and absence of CADs (20 ![]() M),
or 2% DMSO were placed into the cultures and these dishes
were incubated for 24 hours. These cultures were rinsed,
fixed with 10% phosphate-buffered formalin, and stained
with filipin. 10, 11
Slides were examined and photographed with fluorescense
microscopy.
M),
or 2% DMSO were placed into the cultures and these dishes
were incubated for 24 hours. These cultures were rinsed,
fixed with 10% phosphate-buffered formalin, and stained
with filipin. 10, 11
Slides were examined and photographed with fluorescense
microscopy.
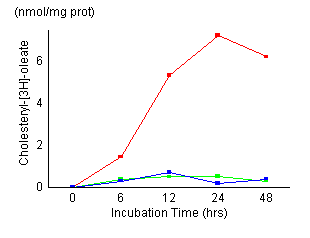
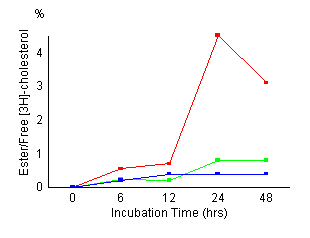
Time
course of cholesterol esterification in control fibroblasts
(red), fibroblasts treated with 20 ![]() M
AY-9944 (green) and NPC fibroblasts (blue). Control
cells and NPC cells were established as described in
Materials and Methods. Cells
were incubated for the indicated time (6, 12, 24, 48
hours) and harvested. Cellular levels of unesterified
and esterified cholesterol were measured as described
in Materials and Methods. The
results represented a mean of triplicates. AY-9944 caused
a marked reduction of cholesterol esterification during
the incubation time as in NPC cells. There were no apparent
morphological changes in the fibroblasts exposed continuously
for 48 hours to 20
M
AY-9944 (green) and NPC fibroblasts (blue). Control
cells and NPC cells were established as described in
Materials and Methods. Cells
were incubated for the indicated time (6, 12, 24, 48
hours) and harvested. Cellular levels of unesterified
and esterified cholesterol were measured as described
in Materials and Methods. The
results represented a mean of triplicates. AY-9944 caused
a marked reduction of cholesterol esterification during
the incubation time as in NPC cells. There were no apparent
morphological changes in the fibroblasts exposed continuously
for 48 hours to 20 ![]() M
AY-9944.
M
AY-9944.
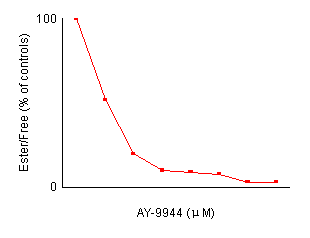
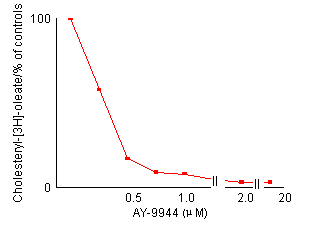
Effects of AY-9944 concentration on the reduction of cholesterol esterification in control fibroblasts. After preincubation with LPDS-supplemented medium for 4 days, non-confluent cells were incubated for 24 hours with fresh medium containing the indicated amount of AY-9944 by the two methods described in Materials and Methods. Each value is a mean of triplicates and is expressed as percentages of the control activity. In both methods, the reduction of cholesterol esterification is dependent an the concentration of AY-9944.
Effects of AY-9944 on cholesterol esterification
[Figures
1 and 2] The control fibroblasts showed
maximum cholesteryl oleate formation at 24 hours
of incubation time, its maximum value was 7.2 nmol/mg prot. NPC cells
showed a marked reduction of cholesteryl oleate formation. Esterification
of LDL-derived cholesterol in fibroblasts treated with AY-9944 reduced
to 0.5 nmol/mg prot at 24 hours of incubation, and the reduced
activity remained almost unchanged during subsequent incubation. Esterification
of non-lipoprotein cholesterol was also reduced as well as LDL-derived
cholesterol. Figure 2 shows the effects of the
concentration of AY-9944 on cholesterol esterification in control fibroblasts.
The reduction of cholesterol esterification depended on the concentration
of AY-9944. A concentration of more than 2.0 ![]() M
was considered to be adequate for the present experiments. Fluorescent
filipin staining (Figure 3) for unesterified cholesterol
confirmed that the addition of AY-9944 to the culture medium substantially
caused an excessive and abnormal accumulation of unesterified cholesterol
in control fibroblasts as in NPC cells.
M
was considered to be adequate for the present experiments. Fluorescent
filipin staining (Figure 3) for unesterified cholesterol
confirmed that the addition of AY-9944 to the culture medium substantially
caused an excessive and abnormal accumulation of unesterified cholesterol
in control fibroblasts as in NPC cells.
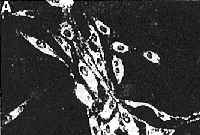



Fluorescent filipin staining for unesterified cholesterol
in control cells (A). NPC cells (B), control cells treated
with 2O ![]() M
AY-9944 (C), and NPC cells treated with 2% DMSO (D).
The procedures of filipin staining were described in
Materials and Methods. Control
fibroblasts (A) showed minimal filipin stained intracellular
unesferified cholesterol. In contrast, NPC fibroblasts
(B) showed numerous filipin-stained, unesterified cholesterol-containing
intracellular inclusions. Fibroblasts treated with AY-9944
(C) also showed an accumulation of unesterifted cholesterol
as wefl as NPC fibroblasts. NPC cells treated with 2%
DMSO decreased their accumulation of unesterified cholesterol.
There were no apparent morphological changes in the
fibroblasts exposed continuously for 4 days to 20
M
AY-9944 (C), and NPC cells treated with 2% DMSO (D).
The procedures of filipin staining were described in
Materials and Methods. Control
fibroblasts (A) showed minimal filipin stained intracellular
unesferified cholesterol. In contrast, NPC fibroblasts
(B) showed numerous filipin-stained, unesterified cholesterol-containing
intracellular inclusions. Fibroblasts treated with AY-9944
(C) also showed an accumulation of unesterifted cholesterol
as wefl as NPC fibroblasts. NPC cells treated with 2%
DMSO decreased their accumulation of unesterified cholesterol.
There were no apparent morphological changes in the
fibroblasts exposed continuously for 4 days to 20 ![]() M
AY-9944 and 2% DMSO. (original magnification x 50)
M
AY-9944 and 2% DMSO. (original magnification x 50)
Effect of other cationic amphiphilic drugs on cholesterol esterification
Using
the fibroblast system as described above, we next examined
eleven other CADs (Table 1). Fibroblasts
were incubated with medium containing each of the drugs
at 20 ![]() M
for 24 hours, then the LDL-derived cholesterol esterirication
and non-lipoprotein cholesterol estetification were
measured, respectively. AD drugs caused a considerable
reduction of cholesterol esterification with both methods,
which were dependent on each concentration (data not
shown). Filipin staining also showed increased intracellular
inclusions in cells treated with each drug (data not
shown).
M
for 24 hours, then the LDL-derived cholesterol esterirication
and non-lipoprotein cholesterol estetification were
measured, respectively. AD drugs caused a considerable
reduction of cholesterol esterification with both methods,
which were dependent on each concentration (data not
shown). Filipin staining also showed increased intracellular
inclusions in cells treated with each drug (data not
shown).
Effects of various cationic amphiphilic drugs on esterification of non-lipoprotein cholesterol and LDL-derived cholesterol in fibroblasts
| [3H]- cholesterol* |
Cholesteryl- [3H]-oleate formation* |
||||
|---|---|---|---|---|---|
| Ester | Free | Ester/Free (%) | |||
| Control | 7.6 | 161.6 | 4.7 | 7.2 | |
| NPC | 0.5 | 137.7 | 0.4 | 0.3 | |
|
|
|||||
| Control | + AY-9944 | 1.3 | 187.5 | 0.7 | 0.5 |
| + Imipramine | 1.3 | 233.8 | 0.6 | 0.7 | |
| + Desipramine | 1.7 | 301.8 | 0.6 | 0.7 | |
| + Trimipramine | 1.4 | 177.2 | 0.8 | 1.1 | |
| + Lofeptamine | 0.9 | 215.6 | 0.4 | 0.8 | |
| + Clomipramine | 1.0 | 125.4 | 0.8 | 1.0 | |
| + Mianserin | 1.1 | 198.2 | 0.6 | 0.8 | |
| + Chlorpromazine | 2.0 | 203.8 | 1.0 | 0.7 | |
| + W-7 | 1.8 | 244.0 | 0.7 | 0.9 | |
| + W-5 | 0.9 | 167.3 | 0.5 | 1.0 | |
| + Dibucaine | 1.5 | 159.2 | 0.9 | 0.6 | |
| + Chloroquine | 3.2 | 189.0 | 1.7 | 1.7 | |
|
After
preincubation in DME medium supplemented with
10% LPDS for 4 days, the cells were fed with
fresh Lipoprotein free medium containing CADs
at a concentration of 20 *expressed as nmol/mg prot/24 hr. |
|||||
After preincubation in DME medium supplemented with
10% LPDS for 4 days, the cells were fed with fresh
Lipoprotein free medium containing CADs at a concentration
of 20 ![]() M
unless otherwise indicated. Cholesteryl ester synthesis
was studied by incubating the cholesterol-depleted
cells with or without IDL (50
M
unless otherwise indicated. Cholesteryl ester synthesis
was studied by incubating the cholesterol-depleted
cells with or without IDL (50 ![]() g/mo
in the medium supplemented with 40
g/mo
in the medium supplemented with 40 ![]() l
of 12.5 mM [3H] oleic acid (200 dpm/pmol)
in 12% (wt/vol) bovine serum albumin for 24 hours,
or in the medium supplemented with 12
l
of 12.5 mM [3H] oleic acid (200 dpm/pmol)
in 12% (wt/vol) bovine serum albumin for 24 hours,
or in the medium supplemented with 12 ![]() of 32 mM [3H]-cholesterol (30 dpm/pmol)
for 24 hours. The cells were harvested and processed
to lipid analysis described in Methods.
The results were expressed as LDL-stimulated cholesterol
[3H] oleate formation, and % of [3H]
cholesteryl esters to free [3H] cholesterol.
Each value is a mean of triplicates.
of 32 mM [3H]-cholesterol (30 dpm/pmol)
for 24 hours. The cells were harvested and processed
to lipid analysis described in Methods.
The results were expressed as LDL-stimulated cholesterol
[3H] oleate formation, and % of [3H]
cholesteryl esters to free [3H] cholesterol.
Each value is a mean of triplicates.
*expressed as nmol/mg prot/24 hr.
Effect of DMSO on cholesterol esterification
Control fibroblasts treated with 2% DMSO (Table
2) showed similar levels of cholesterol esterification.
However, DMSO partially corrected the cholesterol
esterification of NPC fibroblasts and fibroblasts
treated with 20 ![]() M
AY-9944. Filipin staining confirmed that DMSO reduced
unesterified intracellular inclusions in NPC fibroblasts
(Fig 3) and in control cells
treated with AY-9944.
M
AY-9944. Filipin staining confirmed that DMSO reduced
unesterified intracellular inclusions in NPC fibroblasts
(Fig 3) and in control cells
treated with AY-9944.
Effect of DMSO on cholesterol esterification
| [3H] cholesterol Ester/Free (%) |
Cholesteryl- [3H]-oleate formation |
|
|---|---|---|
| Control | 5.0 | 7.2 |
| Control + DMSO | 5.2 | 7.3 |
| NPC | 0.4 | 0.3 |
| NPC + DMSO | 1.5 | 1.7 |
| Control + AY-9944 | 0.5 | 0.5 |
| Control + AY-9944 + DMSO | 1.8 | 1.6 |
AY-9944 was shown to cause a specific reduction of acid sphingomyelinase activity in fibroblasts5 and caused an animal model to mimic Niemann-Pick disease. 6 lmipramine and desipiamine, both tricyclic antidepressants, also caused a severe deficiency of acid sphingomyelinase in murine neuroblastoma and human fibroblasts. 12 Many CADs other than these drugs were reported to have similar effects. 5. CADs which possess a hydrophobic moiety and a hydrophilic region differ widely in their principal pharmacological actions. However, more than 30 CADs have been shown to cause lipidosis in a variety of tissues in vivo and in cell cultures. 13 Although the mechanism as to how a rapid and reversible inactivation of acid sphingomyelinase is caused by CADs remains unexplained, the amphiphilic and cationic properties of these drugs are considered to be required for an inhibitory effect on acid sphingomyelinase activity, and it is suggested that the reduced activity of the acid sphingomyelinase is a general consequence of the action of these CADs. 5 Although the effects of CADs on acid sphingomyelinase had been previously fully investigated,5 the effects of these drugs, except for imipramine,14 on cholesterol esterification had not.
The present data demonstrate that CADs other than imipramine induce a lack of stimulation of cholesteryl ester synthesis by exogenous LDL or non-lipoprotein cholesterol, and an excess intracellular accumulation of unesterified cholesterol, both of which can partially be corrected by the addition of DMSO. These characteristics mimic the phenomena seen in NPC fibroblasts, as previously reported. 1-3 Recently, imipramine has been shown to promote marked alterations of cholesterol esterification resembling those in NPC. 14 In the case of imipramine, the drug could alter the permeability of the lysosomal membrane15, 16 and moderate cholesterol esterification. 14 At present, a possible explanation is difficult in other CADS. However, the mechanism of the effect of CADs on cholesterol esterification may be related to the alteration of membrane permeability, as imipramine did. We consider that it is important to elucidate the mechanism of these drug effects because we have suspected that the pathogenesis of NPC is accompanied in part with the effects of these drugs. 5, 6
Concerning the relationship between cholesterol metabolism and acid sphingomyehnase activity, a close relation exists between the metabolism of sphingomyelin and cholesterol, a common lipid component of biological membranes. Maziere et al17 reported that cholesterol and 7-dehydrocholesterol inhibit the acid sphingomyelinase activity of human fibroblasts. Their investigations suggest that an accumulation of 7-dehydrocholesterol by AY-9944 also can inhibit acid sphingomyelinase activity. This hypothesis was supported by the study18 that the removal of lipoprotein from a medium of NPC results in a complete correction of the partial sphingomyelinase deficiency seen when the cells are cultured in a regular medium containing this fraction. Therefore, a reduction of acid sphingomyelinase activity induced by AY-9944 may be secondarily caused by an excessive intracellular accumulation of unesterified cholesterol, as seen in NPC fibroblasts.
DMSO, because of its dipolar nature, can effectively interact with water, salts, protein, and lipids and readily permeates through membranes and tissues without irreversible damage. 19 These responses reflect a wide diversity of pharmacological and medical applications of DMSO that include analgesic, sedative, anti-inflammatory, diuretic, and vasodilator effects. 19 Sakuragawa, et al20, 21 previously reported that DMSO corrects a partial deficiency of acid sphingomyelinase activity in NPC fibroblasts and the oral administration of DMSO caused clinical improvement, such as a reduction of hepatosplenomegaly and seizure frequency in NPC patients. In the present study, the addition of 2% DMSO to the culture medium partially corrected impaired cholesterol esterification and reversed an excess accumulation of free cholesterol in NPC cells and cells treated with AY-9944 and other CADs. Recently, Mackie, et al22 reported that DMSO moderate cholesterol processing in NPC fibroblasts which were in good agreement with the present results. Furthermore, we have demonstrated that DMSO corrects impaired esterification of cholesterol induced by AY-9944 and other CADs. As stated by Mackie, et al,22 DMSO may alter the permeability and stability of lysosomal membranes and make intracellular unesterified cholesterol soluble, thus increasing acid sphingomyelinase activity.
As described above, it is interesting that both CADs and DMSO may influence membrane function and alter the esterification of cholesterol. Further, CADs could cause a similar condition to that in NPC cells. These characteristics of the effects of AY-9944 or other CADs indicate that normal fibroblasts treated with these drugs may be used as a model of drug-induced Lipidosis of NPC. Also, alteration of the lipoprotein level or intracellular cholesterol level might influence the biochemical18 and probably the clinical manifestation of NPC. Therefore, it is suggested that DMSO may be used in the treatment of NPC patients and reduce the severity or progression of such patients.
This study was supported by a Grant (2-A) from National Center of Neurology and Psychiatry (NCNP) of the Ministry of Health and Welfare Japan. The author is deeply grateful to Prof. Tsugutoshi Aoki, the Second Department of Pediatrics, Toho University School of Medicine, and Dr. Norio Sakuragawa, Chief, Division of Inherited Metabolic Disorders, National Institute of Neuroscience, NCNP, for their kind suggestions and advice on this work.
- Spence MW, Callaham JW. Sphingomyelin-cholesterol lipidosis: the Niemam-Pick group of diseases. In: Scriver CR, Beaudet AL, Sly WS, VaUe D, eds. The metabolic basis of inherited disease. 6th ed. New York: McGraw-Hill, 1989: 1655-76.
- Pentchev PG, Comly ME, Kruth HS, et al. A defect in cholesterol esterification in Niemann-Pick disease (type C) patients. Proc Natl Acad Sci USA 1985;82:8247-51.
- Vanier MT, Wenger DA, Comly MA, et al. Niemann-Pick disease group C: clinical variability and diagnosis based on defective cholesterol esterification. Clin Genet 1988; 33:331-48.
- Schroepfer GJ Jr, Frantz ID Jr. Conversion of delta7-cholesterol-4-C1 4 to cholesterol. J Biol Chem 1961; 236:3137-40.
- Yoshida Y, Arimoto K, Sato M, et al. Reduction of acid sphyngomyelinase activity in human fibroblasts induced by AY-9944 and other cationic amphiphilic drugs. J Biochem 1985;98:1669-79.
- Sakuragawa N, Sakuragawa M, Kuwabara T, et al. Experimental model of Niemann-Pick disease. Sphingomyelinase reduction induced by AY-9944. Science 1977; 196:317-9.
- Sato M, Yoshida Y, Sakuragawa N, Arima M. Effects of dimethyl sulfoxide on sphingomyelinase activities in normal and Niemann-Pick type A, B and C fibroblasts. Biochim Biophys Acta 1988;962:59-65.
- Lowry OH, Rosebrough NJ, Farr AL, Randal RJ. Protein measurement with folin phenol reagent. J Biol Chem 195 1;193:265-75.
- Folch J, Lee M, Sloane-Stanley GH. Simple methods for the isolation and purification of total lipids from animal tissue. J Biol Chem 1954;226:497-509.
- Bomig H, Geyer G. Staining of cholesterol with the fluorescent antibiotic filipin. Acia Histochem 1974; 50:110-5.
- Kruth HS, Vaughan M. Quanfication of low density lipoprotein binding and cholesterol accumulation by single human fibroblasts using fluoresence microscopy. J Lipid Res 1980;21:123-30.
- Albouz S, Boutry JM, Dubois G, et al. Lipid and lysosomal enzymes in human fibroblasts cultured with perhexiline maleate. Naunyn-Schmied Arch Pharmacol 1981;317:173-7.
- Lullman-Rauch R. Drug-induced lysosomal storage disorders. In: Dingle JT, Jacques PT. eds. Lysosomes in biology and pathology. Vol 6. Amsterdam-New York-Oxford: North-Holland, 1979:49-130.
- Rodriguez-Lafrasse C, Rousson R, Bonnet R, et al. Abnormal cholesterol metabolism in imipramine-treated fibroblast culture. Similarities with Niemann-Pick type C disease. Biochem Biophis Acta 1990;1043:123-8.
- Shacoori V, Leray G, Gueble-Val F, Le Treut A, Le Gall JY. Inhibition of (Na,K)-ATPase and Mg-ATPase by a lysosomotropic drug: perhexiline maleate. Res Commun Chem Pathol Pharmacol 1988;59:11796-806.
- Dean RT, Jessup W, Roberts CR. Effects of exogenous amines on mammalian cells, with particular reference to membrane flow. Biocliemistry 1984, 217:27-40.
- Maziere JC, Wolf C, Maziere C, et al. Inhibition of human fibroblasts sphingomyelinase by cholesterol and 7-dehydrdrocholesterol. Biochem Biophys Res Communn 1981;100: 1299-304.
- Thomas GH, Tuck-Muller CM, Miller CS, Reynolds LW. Correction of sphingomyelinase deficiency in Niemann-Pick type C fibroblasts by removal of lipoprotein fraction from culture media. J Inher Metab Dis 1989; 12:139-51.
- Kharasch N, Thyagarajan BS. Structural basis for biological activities of dimethyl sulfoxide. Ann NY Acad Sci 1983; 411:391-402.
- Sakuragawa N, Sato M, Yoshida Y, et al. Effects of dimethyl sulfoxide on sphingomyelinase in cultured human fibroblasts and correction of sphingomyelinase deficiency in fibroblasts from Niemann-Pick patients. Biochem Biophys Res Commun 1985; 126:756-62.
- Sakuragawa N, Sato M, Kamo I, Arima M. Therapeutic effects of dipolar aprotic substances on Niemann-Pick cells. Acta Paediatr Jpn (Tokyo) 1987;29:433-40.
- Mackie EJB, Dwyer NK, Vanier MT, et al. Type C Niemann-Pick disease: dimethyl sulfoxide moderates abnormal LDL-cholesterol processing in mutant fibroblasts. Biochem Biophys Acta 1989;1006:219-26.
Brain Development 1991;13:115-20
From the Division of Inherited Metabolic Disorders, National Institute of Neuroscience, National Center of Neurology and Psychiatry, Kodaira, Tokyo.
Correspondence address: Dr. Norio Sakuragawa, Division of Inherited Metabolic Disorders, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1, Ogawahigashi-cho, Kodaira, Tokyo, 187, Japan.
DMSO Organization wishes to thank the publishers of Brain Development for allowing this article to be placed on our World Wide Web site. The publisher retains all copyright. To copy any portion of this article, obtain permission from the publisher.
© 2001-2022 All rights reserved